Abstract
Introduction CART cell therapy has improved OS in r/r LBCL, but less than half of pts are long term survivors. Our goal was to evaluate the association of two previously developed prognostic scores, the CLL-CI and IPI (Gordon, 2021 and Sehn, 2007) with survival after CART.
Methods Pts with r/r LBCL treated with standard of care CART cell therapy at the MD Anderson Cancer Center between 2017-2021 were retrospectively analyzed. Prognostic factors were assessed prior to apheresis. Comorbidities were assessed using the CLL-CI, a three-factor comorbidity score which consists of vascular, endocrine and upper gastrointestinal comorbidities as categories. Each category receives 1-point if any condition is present for a maximum score of 3. A score of 0 is low risk, 1 intermediate risk and 2-3 high-risk. Survival was estimated using the Kaplan Meier method and cause specific hazard functions, with difference assessed by log-rank. Multivariable Cox regression was used to model OS.
Results In total, 218 pts were included. Median age was 61 years (range, 18-89). Median prior lines of therapy were 3 (range, 1-11). The majority of pts, 202 (93%) were treated with axicabtagene ciloleucel and 16 (7%) were treated with tisagenlecleucel. IPI score was 0-1 in 13%, 2 in 25%, 3 in 31% and 4-5 in 31%. Except for age >60, all components of the IPI were significantly associated with OS. Age >70 was associated with shorter OS. CLL-CI score was 0 in 44%, 1 in 36% and 2-3 in 21%.
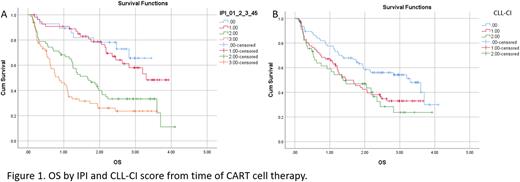
IPI score was significantly associated with OS (Fig 1A). Median OS was not reached (NR), 3.3, 1.7 and 0.9 years in IPI 0-1, 2, 3 and 4-5, respectively (p<.0001). CLL-CI score was also associated with OS, median 3.3, 1.5 and 1.3 years with a score of 0, 1 and 2-3, respectively (Fig 1B; p=.01). In multivariable models both IPI (HR 1.8, p=<.0001) and CLL-CI (HR 1.3, p=.03) were independently associated with OS.
We also performed cause specific survival analyses by treating other causes of death as competing risks. The IPI score was not associated with toxic/infectious death (p=0.24) while CLL-CI score was (p=.004). Death due to toxic/infectious complications at 1 year occurred in 0%, 6% and 18% of pts with CLL-CI score of 0, 1 and 2-3, respectively. Interestingly, CLL-CI was not significantly associated with grade 3-4 cytokine release syndrome (p=.36). There was a trend towards higher rates of grade 3-4 immune effector cell associated neurotoxicity syndrome with high CLL-CI (p=.07).
We also evaluated cause specific survival with death due to progressive disease as the outcome of interest. Median progressive disease specific survival was NR, NR, 2.2 and 1.1 years in IPI 0-1, 2, 3 and 4-5, respectively (p<.0001). Progressive disease specific survival was not associated with CLL-CI (p=.15).
We further evaluated the association of toxic/infectious death, age and comorbidity. A total of 21 pts (9.6%) died within 1 year of CART due to toxic/infectious complications; 38% (N=8) of all toxic/infectious deaths were in pts with a CLL-CI score of 2-3 who were >70 years old. In pts >70 years, the rate of death at 1 year due to toxic/infectious complications was 0%, 7% and 39% in pts with a low, intermediate and high CLL-CI score, respectively (p=.009). In pts ≤70 years death at 1 year due to toxic/infectious complications occurred in 0%, 5% and 4% in CLL-CI low, intermediate and high, respectively (p=.031).
Finally, we assessed the test characteristics of the IPI and CLL-CI to predict death at 1 year. Using receiver operator characteristics to assess death due to toxic/infectious, the AUC for the CLL-CI was .82 (95% CI, .73-.91) which is comparable to the AUC for IPI which was .75 (95% CI, .67-.82) when assessing death due to progressive disease.
Discussion The IPI and CLL-CI, assessed prior to CART, are associated with survival in r/r LBCL. IPI is more strongly associated with death due to progressive disease and the CLL-CI with death due to toxic/infectious complications-particularly in older pts (age >70 years). It is interesting that a score previously validated in CLL also applies to LBCL and suggests vascular, moderate/severe endocrine, moderate/severe upper gastrointestinal comorbidity correlates with survival in pts with different malignancies. Future research could further elucidate the pathophysiology driving the increased risk in pts with these particular comorbidities. And mitigating toxicity and infection in high CLL-CI pts treated with CART should also be a focus of future research
Disclosures
Ahmed:Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Merck: Research Funding; Myeloid Therapeutics: Consultancy. Steiner:BMS: Research Funding; Seagen: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Strati:Roche Genentech: Consultancy; Sobi: Research Funding; ALX Oncology: Research Funding; Astrazeneca Acerta: Research Funding; Kite Gilead: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Hutchinson MediPharma: Consultancy. Westin:Iksuda: Consultancy; Novartis: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Merck: Consultancy; Calithera: Consultancy, Research Funding; Abbvie/GenMab: Consultancy; MonteRosa: Consultancy; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; SeaGen: Consultancy. Kebriaei:Amgen: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Ziopharm: Research Funding; Jazz: Consultancy. Shpall:Bayer: Honoraria; adaptimmune: Consultancy; axio: Consultancy; Navan: Consultancy; Takeda: Patents & Royalties; Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; Affimed: Other: License agreement. Neelapu:Bluebird Bio: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Medscape: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Aptitude Health: Consultancy, Research Funding; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Incyte: Consultancy, Honoraria, Other: Personal fees; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Acerta: Research Funding; Bio Ascend: Consultancy, Honoraria; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal